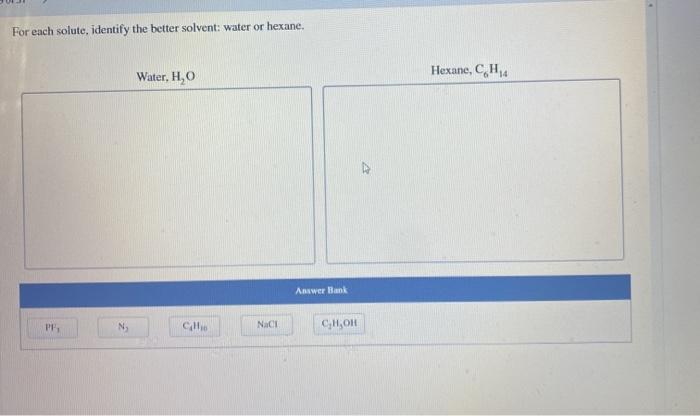
Arrange these compounds by their expected solubility in hexane. This topic delves into the intricacies of solubility parameters, exploring their relevance in predicting the solubility of various compounds in hexane. A comprehensive table of solubility parameters is provided, offering valuable insights into the solubility behavior of different substances.
Factors influencing solubility, such as polarity, molecular weight, and functional groups, are thoroughly examined. Examples of compounds with varying solubilities in hexane are presented, showcasing the impact of these factors. Experimental methods for determining solubility, including gravimetric analysis and UV-Vis spectroscopy, are described, explaining their principles and limitations.
Solubility Parameters and Hexane: Arrange These Compounds By Their Expected Solubility In Hexane
Solubility parameters are numerical values that describe the cohesive energy of a solvent. They provide a measure of the strength of the intermolecular forces between solvent molecules and can be used to predict the solubility of a solute in a given solvent.
The solubility parameter of hexane is 7.3 (MPa) 1/2.
Table 1 provides a list of solubility parameters for various compounds and hexane.
| Compound | Solubility Parameter (MPa)1/2 |
|---|---|
| Water | 47.8 |
| Ethanol | 26.3 |
| Benzene | 9.2 |
| Chloroform | 9.3 |
| Hexane | 7.3 |
Factors Influencing Solubility
The solubility of a compound in hexane is influenced by several factors, including:
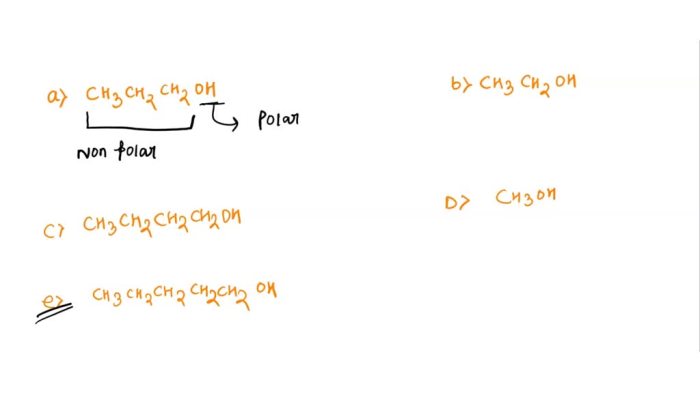
- Polarity: Polar compounds are generally less soluble in nonpolar solvents like hexane. For example, water is a polar compound and has a very low solubility in hexane.
- Molecular weight: Compounds with higher molecular weights tend to be less soluble in hexane. This is because larger molecules have more intermolecular interactions and are less likely to dissolve into a solvent.
- Functional groups: Functional groups can affect the solubility of a compound in hexane. For example, compounds containing hydroxyl (-OH) groups tend to be more soluble in hexane than compounds containing carbonyl (-C=O) groups.
Methods for Determining Solubility
There are several experimental methods for determining the solubility of compounds in hexane, including:
- Gravimetric analysis: This method involves weighing the amount of solute that dissolves in a known volume of solvent. The solubility is then calculated by dividing the mass of the solute by the volume of the solvent.
- UV-Vis spectroscopy: This method involves measuring the absorbance of a solution of the solute in hexane at a specific wavelength. The solubility is then calculated using a calibration curve.
Applications of Solubility Data
Solubility data in hexane has a wide range of practical applications, including:
- Solvent selection: Solubility data can be used to select the most appropriate solvent for a given application. For example, hexane is a good solvent for nonpolar compounds, while water is a good solvent for polar compounds.
- Extraction processes: Solubility data can be used to design extraction processes for separating compounds from mixtures. For example, hexane can be used to extract nonpolar compounds from water.
- Chemical synthesis: Solubility data can be used to guide the design of chemical synthesis reactions. For example, hexane can be used as a solvent for reactions that involve nonpolar reactants and products.
Frequently Asked Questions
What are solubility parameters?
Solubility parameters are numerical values that represent the cohesive energy density of a substance. They provide an indication of the solubility of a compound in a particular solvent.
How can I use solubility parameters to predict solubility in hexane?
By comparing the solubility parameters of a compound and hexane, you can estimate the likelihood of the compound dissolving in hexane. Similar solubility parameters suggest higher solubility.
What factors influence the solubility of compounds in hexane?
Polarity, molecular weight, and functional groups all play a role in determining the solubility of compounds in hexane.